In this podcast i discuss the following points raised by Eveline from Delft University of Technology (original text):
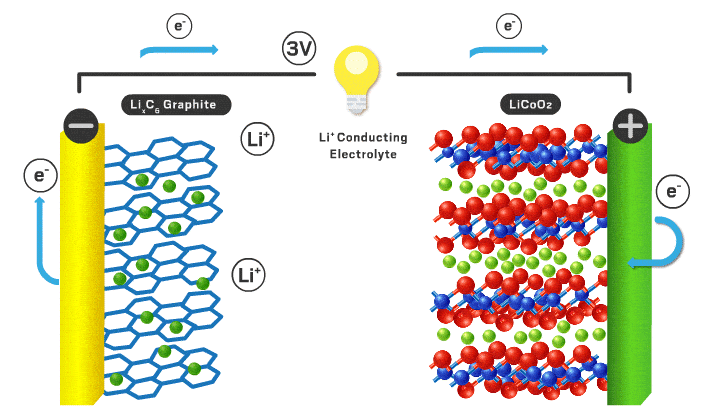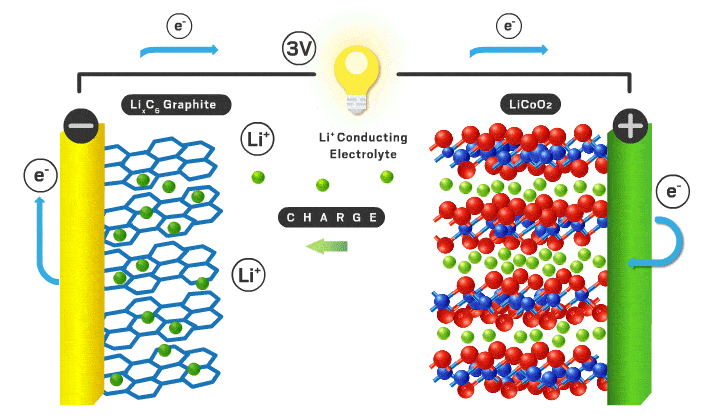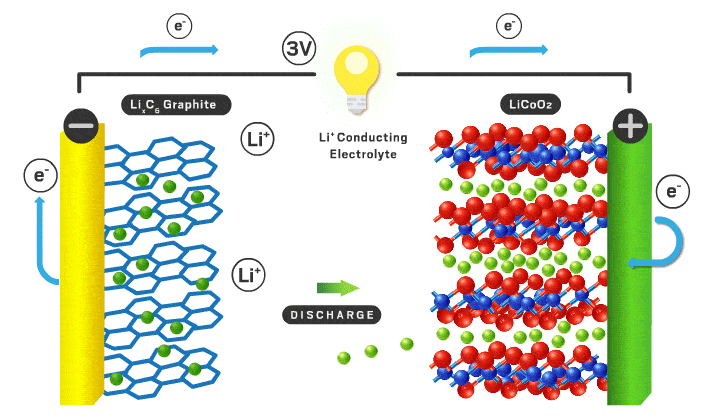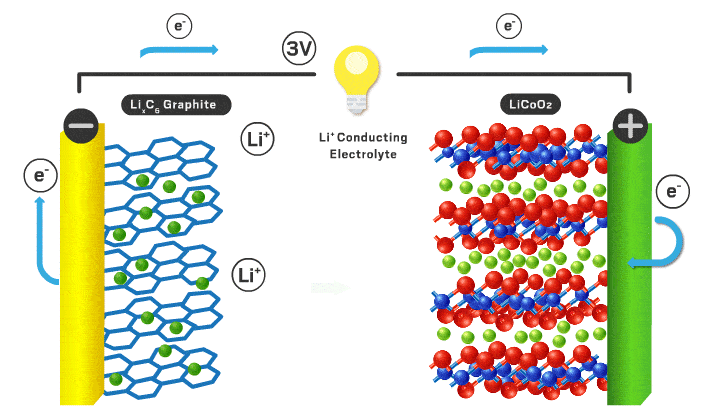
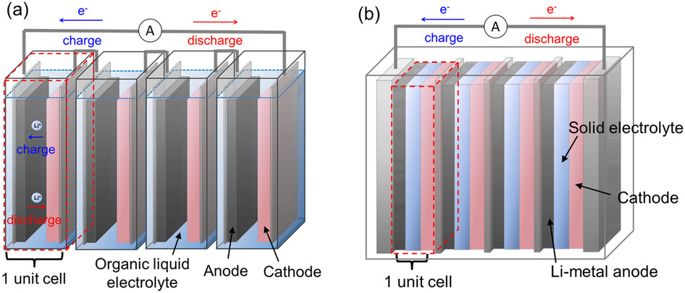
In my group we do a lot of work with solid state inorganic electrolytes. I would be extremely interested in your view about the scale up and economics of such systems! For example, in your last Podcast you describe how the LPS/Polymer cells are made.
1. How could such processes be implement on a larger scale and for larger batteries?
2. And if two polymer interfaces are needed, what is the benefit compared to a composite polymer electrolyte with inorganic fillers?
3. And then, compared to standard lithium-ion, is it even possible that the technology could ever compete economically?